Abstract
Introduction Venetoclax (Ven) in combination with hypomethylating agents (HMA) is frequently used for treatment of relapsed/refractory AML patients in routine practice. However, limited data exists on molecular predictors of response and survival following Ven+HMA therapy in the relapsed/refractory setting. A prior retrospective study demonstrated superior response with TET2and ASXL1 mutations (AJH, 2019). Accordingly, our primary objective was to determine the impact of mutations on response and survival in relapsed/refractory AML patients receiving Ven + HMA.
Methods Patients with relapsed/refractory AML, excluding post-transplant relapse, receiving Ven+HMA outside clinical trials at the Mayo Clinic were retrospectively recruited after institutional review board approval. Cytogenetic and molecular studies were performed at the time of AML diagnosis by conventional karyotype, and next-generation sequencing (42-gene panel), respectively. All patients received either azacitidine 75 mg/m2 days 1-7 or decitabine 20 mg/m2 days 1-5 with Ven dose adjusted based on azole antifungal prophylaxis. Response was assessed according to the 2017 European Leukemia Net (ELN) criteria.
Patient characteristics
87 relapsed/refractory AML patients (median age 64 years, 62% male, 51% de novo) received Ven+HMA. ELN cytogenetic risk (n=77) included favorable (1%, n=1), intermediate (57%, n=44) or adverse (42%, n=32). Mutations involved TP53 in 20 patients (23%), ASXL1 in 19 (22%), IDH1/IDH2 in 12 (14%), TET2 in 11 (13%), K/NRAS in 11 (13%), NPM1 in 9 (10%), and FLT3-ITD in 7 (8%). Frequently administered prior therapies included 7+3 (n=51), CPX-351 (n=17), enasidenib /ivosidenib (n=6). Prior HMA was documented in 18 (21%) patients.
71 (82%) patients received decitabine and the remainder azacitidine with median Ven dose of 100 mg for a median of 2 cycles. Subsequent targeted therapies following Ven+HMA included enasidenib (n=3) and gilteritinib (n=2).
Predictors of response
18 (21%) patients achieved complete remission (CR), 19 (22%) CR with incomplete hematological recovery (CRi), resulting in CR/CRi in 37 (43%). In univariate analysis, age > 65 years (CR/CRi, 61% vs 29%, p=0.003) presence of IDH1/2 (75% vs 37%, p=0.01) and ASXL1 mutations (68% vs 35%, p=0.01) were associated with favorable response; adverse karyotype (25% vs 58%, p=0.004), and presence of TP53 mutations (25% vs 48%, p=0.06) predicted inferior response. Presence of FLT3-ITD (71% vs 40%; p=0.11) NPM1 (67% vs 40%, p=0.12) and TET2 mutations (64% vs 39%, p=0.13) were borderline significant. In multivariable analysis, presence of ASXL1 mutations (OR 3.4) and absence of adverse karyotype (OR 4.5) remained independent predictors of favorable response.
Predictors of survival
At a median follow up of 5.6 months, 69 (79%) patients have died and 20 (23%) underwent allogeneic transplant. Post-Ven+HMA median overall survival was 5.9 months (CI, 2.5-14.5 months) and longer in transplanted patients (38.7 vs 5.1 months, p<0.0001).
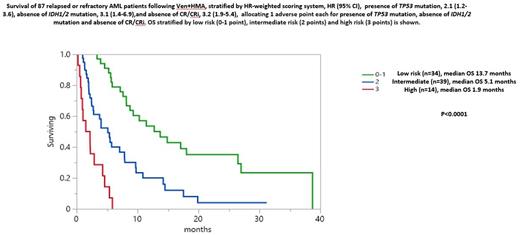
Univariate analysis identified CR/CRi (p<0.0001), IDH1/2 mutations (p=0.002) as favorable, and TP53 (p=0.005), and adverse karyotype (p=0.04) as unfavorable risk factors for survival. Of note, two IDH2 mutated patients on enasidenib following Ven+HMA remain alive at 27 and 13 months, respectively. Despite higher CR/CRi, presence of ASXL1 mutation did not impact survival. Multivariable analysis confirmed the negative survival impact of not achieving CR/CRi (HR 3.1, 95% CI 1.9-5.4, p<0.001), absence of IDH1/2 (HR 3.1, 95% CI 1.4-6.9, p=0.01), and presence of TP53 mutations (HR 2.1, 95% CI 1.2-3.6, p=0.01). Accordingly, a three-tiered model was generated by allocating 1 adverse point each for absence of CR/CRi, absence of IDH1/2 and presence of TP53 mutations, resulting in low (0-1 point, n=34, 13.7 months), intermediate (2 points, n=39, 5.1 months) and high risk (3 points, n=14, 1.9 months) categories (p<0.0001) (Figure) which remained applicable in non-transplanted patients (9.2 vs 4.0 vs 1.9 months, p<0.0001).
Conclusions The current study identifies presence of ASXL1 mutation and absence of adverse karyotype as predictors of superior response. Survival was positively influenced by presence of IDH1/2, absence of TP53 mutations and achievement of CR/CRi. Our findings require validation in prospective series, which should also take into consideration the survival impact of subsequent targeted therapies.
Disclosures
Al-Kali:Astex: Other: research support to institution. Mangaonkar:Bristol Myers Squibb: Research Funding. Litzow:Abbvie: Research Funding; Amgen: Research Funding; Astellas: Research Funding; Novartis: Research Funding; Syndax: Research Funding; Jazz: Consultancy; Actinium: Research Funding; Pluristem: Research Funding; Biosight: Other: Data Monitoring Board. Shah:Astellas: Research Funding; Celgene: Research Funding; Marker Therapeutics: Research Funding. Patnaik:Kura Oncology, Stem Line Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal